Abstract
Introduction
In the setting of clinical trials Venetoclax (VEN) combined with hypomethylating agents (HMAs) has shown fair activity in Relapsed/Refractory (R/R) AML and impressive results in newly diagnosed (ND) elderly AML patients. However, no clear guidelines are available on real-life management, especially in the outpatient setting. This study involving AML patients treated with VEN combined with HMAs aims to amelioratate physicians' knowledge about the administration of these regimens.
Methods
This is a single-center retrospective study involving AML patients treated with Venetoclax combined with HMAs. Data were collected in accordance with GCP and Helsinky declaration. Adverse events (AEs) were graded according to CTCAE v4.03. Survival is estimated with Kaplan-Meyer method.
Results
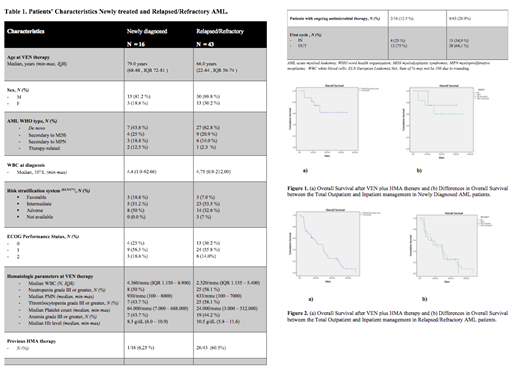
A total of 59 AML patients, 43 R/R and 16 ND, have been treated with VEN plus HMAs from March 2018 to June 2021 and completed at least 1 therapy course (range 1-9, median 2, IQR 1.0 - 4.0). The median age was 70 (range 22-88) years and 15.3 % had a documented ECOG score greater than 1. VEN was combined with azacitidine in 35/59 (59.3 %) patients and with decitabine in 22/59 (37.3 %) patients (Table 1: patient and disease characteristics).
The majority of patients (40/59, 67.8 %) (28/43 R/R, 12/16 ND) received the first cycle as out-patients, 19 out of 59 (32.2 %) patients were hospitalized and used as control. No significant differences with regards to disease and patients' characteristics were observed between in- and out-patients. During the ramp-up phase only 2 cases of tumor lysis syndrome (TLS) and 5 AEs were documented. During the first course, a total of 54 AEs were recorded and experienced by 16 over 19 hospitalized patients (84.2 %) and 20 over 40 outpatients (50 %). The 30-day and 60-day mortality were 2.5% (1/40) and 20 % (8/40), respectively, among patients receiving first course as out-patents, comparable to those documented in hospitalized patients.
Overall, we reported 118 AEs, of which 74 were grade III-IV and the most common were hematological 23/74 (31.1 %) or infective 41/74 (55.4 %). Regarding infections, at least one bacterial infection was experienced in 10/40 (25%) and 12/19 (63.1%) patients of the outpatient and hospitalized cohorts, respectively (p = 0.009, IC 1.37 - 19.74, OR 4.98). Pneumonia and sepsis (13 and 18 cases) were the most frequent infections. Sepsis incidence was higher among hospitalized patients (13/19, 68.4 %, vs 5/40, 12.5 %, p = 0.000029). No significant difference in infective risk was documented between R/R and ND patients (65.1 vs 50 %).
Thirty-two out of 59 (54.2 %) patients experienced at least one VEN withdrawal due to treatment toxicity. Twenty out of 43 AEs requiring VEN suspensions occurred during the first cycle. Patients treated in-patient showed the tendency for a higher probability to suspend therapy due to treatment toxicity (10/19 IN vs 10/40 OUT, p = 0.04). While twenty-three out of 59 (38.9 %) patients were hospitalized for treatment complications at least once, the average number of days spent in hospital was significantly different between patients receiving the first course as outpatients as compared to those who were hospitalized (5.9 vs 39.7, respectively, p < 0.0001).
With a median follow-up of 117 days (IQR 92 - 173.75) in ND patients the Overall Response Rate (ORR), defined as CR + CRi + HI, was 62.5 % (10/16), with a CR/CRi rate of 50 % (8/16) and a median OS of 247 days (95% C.I. 177.71- 316.58)(Fig 1a). In the R/R setting the ORR rate was 41.8 % (18/43), with a CR/CRi rate of 25.6 % (11/43) and a median OS of 219 days (95% C.I. 91.8 - 346.2) (Fig 2a). No differences in OS were documented between patients who underwent VEN plus HMAs outpatient and patients who underwent the first cycle hospitalized (p = 0,38) (Fig. 1b-2b).
Conclusions
With the limitations of a single-center retrospective study, our real-life data indicate that VEN plus HMAs is feasible in an outpatient management, with minimal TLS rate and no limiting toxicities. Of note, infections rate was acceptable, bacterial infections and sepsis risk were lower in outpatients than in hospitalized patients. There was no significant impact of the outpatient management on treatment effectiveness, with data in line with published AML cohorts. Further studies evaluating the clinical, social and economic impact of outpatient VEN-based treatments are highly warranted.
Papayannidis: Pfizer, Amgen, Novartis: Honoraria. Cavo: Adaptive Biotechnologies: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Accommodations, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Speakers Bureau; Novartis: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol-Myers Squib: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Curti: Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees.